
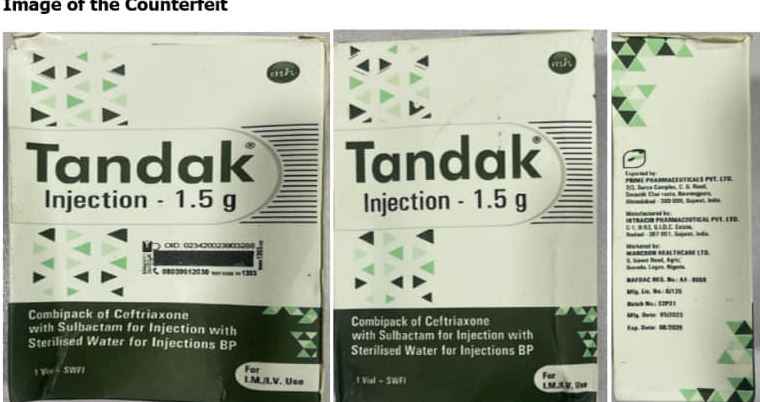
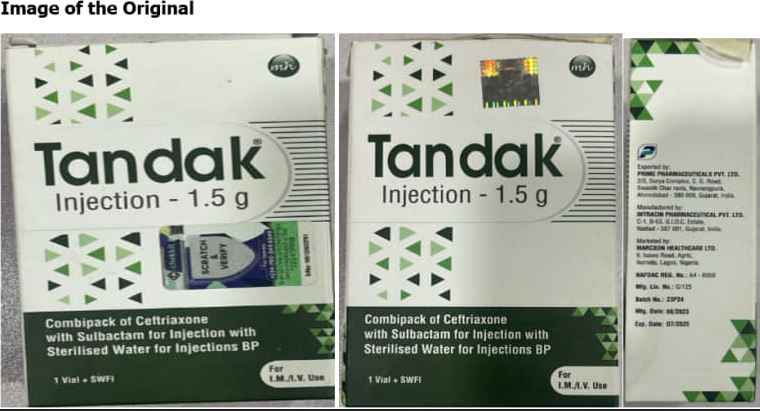
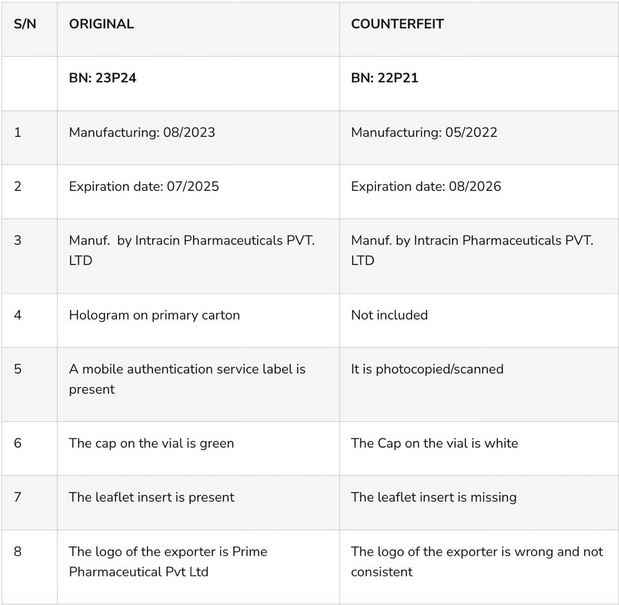
The National Agency for Food and Drug Administration and Control (NAFDAC) has sounded the alarm regarding the sale of counterfeit TANDAK injection 1.5g powder and water for injection, originating from Intracin Pharmaceuticals PVT. LTD in Gujarat, India.
In a press release issued on Wednesday, April 10, NAFDAC disclosed that the illicit product surfaced in Gombe State, Nigeria, prompting swift action by Marcson Healthcare Ltd., the Marketing Authorization Holder (MAH), who reported the discovery to the agency.
TANDAK® injection, a crucial medication comprising Ceftriaxone 1000mg and Sulbactam 500mg, serves as a potent remedy against various bacterial infections. It operates by inhibiting the growth and spread of harmful microorganisms. However, NAFDAC emphasizes that the administration of Ceftriaxone+Sulbactam 1000mg/500mg Injection should strictly occur under the supervision of qualified healthcare professionals.
The proliferation of counterfeit pharmaceuticals poses grave risks to public health, as these products bypass regulatory standards, compromising safety, quality, and efficacy. Recognizing the urgent need to safeguard public health, NAFDAC has issued directives to its Zonal Directors and State Coordinators to conduct rigorous surveillance and eliminate counterfeit TANDAK products from circulation across zones and states.
Healthcare practitioners and consumers are urged to remain vigilant and promptly report any suspicions regarding the sale of substandard or falsified medicines or medical devices to the nearest NAFDAC office. Alternatively, individuals can reach out via the toll-free hotline at 0800-162-3322 or send an email to [email protected].
In light of this development, NAFDAC underscores its commitment to upholding stringent regulatory standards and ensuring the availability of safe and efficacious pharmaceuticals for all Nigerians. Vigilance and collaborative efforts within the healthcare community are pivotal in combating the menace of counterfeit drugs and safeguarding public health.
| Posted: at | |





 TRENDING GISTS
TRENDING GISTS  UK Police Recover Body Of 16Yr Old British-Nigerian Boy Who Drowned In A Lake
UK Police Recover Body Of 16Yr Old British-Nigerian Boy Who Drowned In A Lake PHOTOS: Young Woman Brutally Murdered By Suspected Ritualists, Priv@te Part Removed In Ogun
PHOTOS: Young Woman Brutally Murdered By Suspected Ritualists, Priv@te Part Removed In Ogun Lovely Video Of Singer Davido And His Wife, Chioma Vibing To His Song
Lovely Video Of Singer Davido And His Wife, Chioma Vibing To His Song Woman Slams Her Husband For Allegedly Having Unprotected S3x With Random Girls After Publicly Apologizing To Her
Woman Slams Her Husband For Allegedly Having Unprotected S3x With Random Girls After Publicly Apologizing To Her Actress, Laide Bakare Crowned UN Ambassador Of Peace
Actress, Laide Bakare Crowned UN Ambassador Of Peace Actress, Uche Montana Celebrates Birthday With New Stunning Photos
Actress, Uche Montana Celebrates Birthday With New Stunning Photos VIDEO: President Tinubu Conferred With a Chieftaincy Title in Anambra State
VIDEO: President Tinubu Conferred With a Chieftaincy Title in Anambra State Passengers Killed, Vehicles Set Ablaze As Gunmen Attack Commercial Bus in Imo
Passengers Killed, Vehicles Set Ablaze As Gunmen Attack Commercial Bus in Imo Fan Gushes As MC Oluomo’s Daughter Gets Engaged
Fan Gushes As MC Oluomo’s Daughter Gets Engaged